श्री चित्रा तिरुनाल आयुर्विज्ञान और प्रौद्योगिकी संस्थान, त्रिवेंद्रम
विज्ञान एवं प्रौद्योगिकी विभाग, भारत सरकार के अधीन एक राष्ट्रीय महत्व का संस्थान
Sree Chitra Tirunal Institute for Medical Sciences and Technology, Trivandrum
An Institution of National Importance, Department of Science and Technology, Govt. of India





Projects
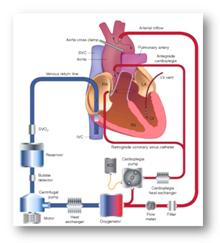
Centrifugal Blood Pump with Blood Flowmeter
- Extracorporeal Cardiopulmonary Bypass is a technique where the patient heart has to be stopped or bypassed during open heart surgery.
- The functions of the heart and lungs will be replaced by blood pump and oxygenator respectively.
- Roller pumps are cost effective for bypass, but causes blood damage.
- Centrifugal blood pump reduces blood damage substantially. Blood flowmeter is used for measuring the flow output from the pump
Phase Principal Investigator:
Mr. Vinodkumar V
Principal Clinical Investigator:
Dr. Vivek V Pillai
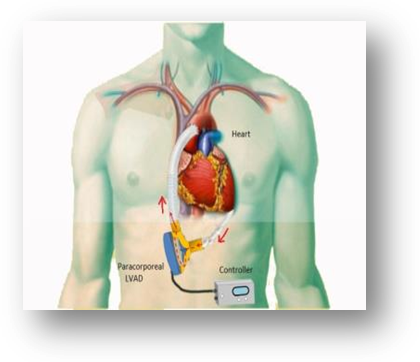
Para-Corporeal Left Ventricular Assist Device
- A mechanical pump used to support heart function in patients who have weakened hearts.
- The device takes blood from the left ventricle of the heart and helps pump it to the body and vital organs, just as a healthy heart would.
- This can be used in patients waiting for heart replacement or patients having problems during surgery where heart will recover if it is allowed to relax for few days.
Phase Principal Investigator:
Mr. D. S. Nagesh
Principal Clinical Investigator:
Dr. K Jayakumar
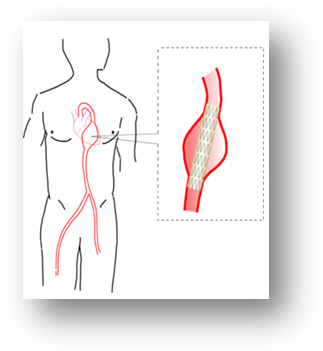
Aortic Stent for Endovascular treatment of Thoracic Aortic Aneurysm
- Used as an alternative to conventional surgical repair of aortic aneurysms (localized blood filled balloon like bulge in the wall of large arteries).
- The device is tubular fabric structure with metal reinforcement which can be delivered to the site using catheters
- The patient recovers fast and it requires only a shorter period of hospital stay.
Phase Principal Investigator:
Mr. Sujesh S
Principal Clinical Investigator:
Dr. Kapilamoorthy T R
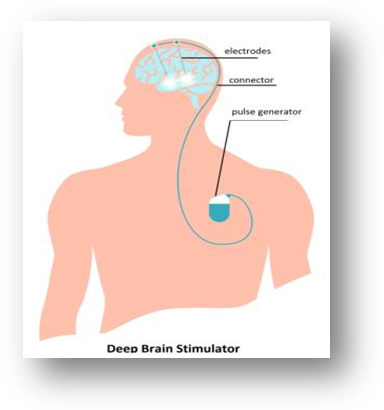
Deep Brain Stimulator System for Movement Disorders
- Parkinson's disease (PD) patients suffer from a variety of movement related disorders such as tremors, stiffness of limbs, slowness of movements and poor balance.
- Low energy electrical stimulation of certain regions of brain using electrodes implanted in the brain controls these symptoms.
- Deep Brain Stimulator employs a pulse generator and a set of platinum electrodes to achieve the required effects.
Phase Principal Investigator:
Mr. Muraleedharan C.V.
Principal Clinical Investigator:
Dr. Asha Kishore
Leukocyte (WBC) Reduction Filter
- Presence of White Blood Cells (WBC) in transfused blood creates many complications to the recipients.
- Leuko-reduction is the removal of white blood cells (or leukocytes) from the blood or blood components supplied for blood transfusion.
- The device employs special membranes which attracts and traps WBC as blood passes through it.
Phase Principal Investigator:
Dr. P Ramesh
Principal Clinical Investigator:
Dr. Debasish Gupta
Bio prosthetic Heart Valve
- Artificial heart valves are devices used for replacing diseased / damaged natural valves of the heart.
- They function as check valves which ensure unidirectional flow of blood in the heart and circulatory system
- Bio prosthetic heart valves are artificial valves made from materials of biological origin and are preferred in elderly patients.
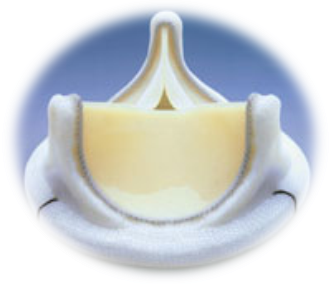
Phase Principal Investigator:
Dr. P R Umashankar
Principal Clinical Investigator:
Dr. Vivek V Pillai
Bioactive inter-vertebral spacers for lumbar fusion
- Instability of lower spine vertebrae due to spondylosis (spinal degeneration) and spondylitis (inflammatory arthritis that affects the spine) often lead to crippling pain.
- Pain relief is achieved surgically by fusing the unstable portion of the spine or immobilizing the vertebral motion segment
- The device helps in joining vertebral bodies through natural healing
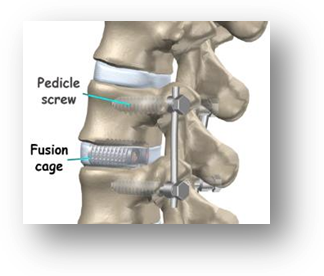
Phase Principal Investigator:
Dr. Manoj Komath
Principal Clinical Investigator:
Dr. Prakash Nair
Bioactive Material Platform for Drug Delivery in bone
- Bone and joint infections (Osteomyelitis) remain one of the most dreaded complications of orthopaedic surgery.
- Bacteria adhere to implants, especially metals, making eradication of infection even more difficult.
- Project aims at the development of a bioactive material platform having a novel bioceramic composition, which has tuned porosity and osteoconductivity
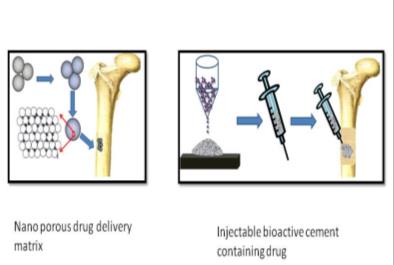
Phase Principal Investigator:
Dr. Harikrishna Varma
Principal Clinical Investigator:
Dr. Easwer H V
Intracranial Electrodes for use in acute and chronic Electro-Corticography for periods up to 15 days.
- Epileptic seizures are the result of excessive and abnormal nerve cell activity in the certain regions of brain.
- Intracranial electrodes are used for locating the epileptogenic regions of brain. They help to locate the regions that need to be removed by surgery for controlling epilepsy.
- The device employ platinum electrodes with platinium - irridium alloy connection wires embedded in silicone rubber.
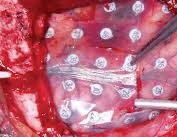
Phase Principal Investigator:
Mr. Jithin Krishnan
Principal Clinical Investigator:
Dr. Ramshekar Menon
Optical Peripheral Nerve Stimulator
- Standard approach to stimulating human nerve cells uses electrical current.
- Optical nerve stimulation overcomes many of the problems like pain associated with electrical stimulation: it requires no physical contact with the nerve cells.
- Light source can be tuned to precisely hit a small desired area.
- The project will focus on the feasibility of optical neural stimulation
Phase Principal Investigator:
Dr. R. S. Jayasree
Blood Derived Products
- Products extracted from human blood and meant for various therapeutic applications
- These include albumin, Factor VIII and Intravenous Immunoglobulin (IVIG).
- The products will be validated to meet the requirements of European / Indian Pharmacopoeia
Phase Principal Investigator:
Dr. Lissy Krishnan
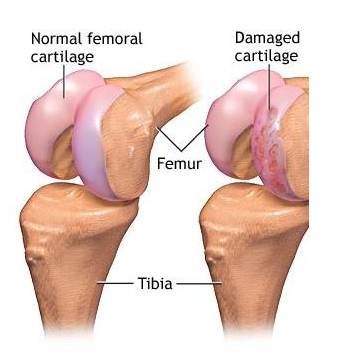
An injectable hydrogel for repair of cartilage injury and growth plate defects
- The incidence of cartilage injury or damage is quite common and may be traumatic or degenerative.
- The aim of the project is to develop injectable hydrogel for repairing damaged cartilages.
- The gel can also be used as a vehicle to transport cartilage producing cells to the site.
Phase Principal Investigator:
Dr. Prabha D.Nair
Principal Clinical Investigator:
Dr. Vrisha Madhuri
3D Printing of Liver Tissue Constructs for In vitro Hepatotoxicity Testing
- The evaluation of potential liver toxicity represents a crucial step in the development of new drugs.
- Laboratory animals are used for carrying out these tests.
- The 3D liver constructs can be used for preliminary screening of drugs for their liver toxicity and help reduce the use of laboratory animals for drug toxicity assays.
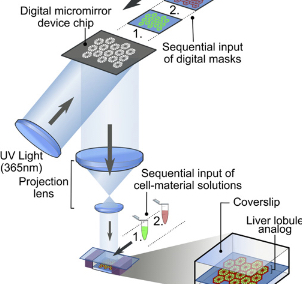
Phase Principal Investigator:
Dr. Anilkumar P. R.
Assay Platform and Sensing Device for PT / INR Monitoring
- Most of the blood vessel disease patients and those with cardiovascular implants, management need continuous and lifelong use of anticoagulant and need a close monitoring of blood clotting time (Prothrombin Time-PT) regularly.
- A home care monitoring system is planned in this project.

Phase Principal Investigator:
Dr. Anugya Bhatt
Principal Clinical Investigator:
Dr. Harikrishnan S
Chitosan / Alginate based Polymeric Wound Dressings for Controlled Antibiotic Delivery
- Wound infection is a major concern in wound management
- The polymeric wound dressing would be capable of in situ loading of antibiotics in it.
- This will be more appropriate as it gives the clinician the freedom to choose the drug of his / her choice

Phase Principal Investigator:
Dr. Rekha M. R.
Fabrication of a wound healing matrix from porcine cholecystic - extracellular matrix
- The extra cellular matrix, from tissues of animal origin after removal of unwanted cells, has a rich load of natural biomolecules that promote tissue regeneration.
- The objective of the project is to fabricate a matrix from porcine tissues.
- The extracellular matrix from gall bladder will be developed for wound healing applications.
Phase Principal Investigator:
Dr. T.V. Anil Kumar
Lint free absorbent dressing for surgical and highly exudating chronic wounds
- The project aims at development of a non - toxic medical dressing having a high fluid holding capacity.
- It will be fast wicking and lint free, even when cut or trimmed.
- The medical dressing will have application in certain highly exudating chronic wounds.

Phase Principal Investigator:
Dr. Lynda V Thomas
Point of Care Devices for Infectious Diseases
- Loop mediated isothermal amplification (LAMP) is a nucleic acid amplification technique which could be carried out using single temperature reaction set up.
- The project attempts to develop a point of care device for detection of tuberculosis using the technique

Phase Principal Investigator:
Dr. Anoop Kumar T
Alginate scaffold with recombinant growth factors for enhanced wound healing
- Growth factors are substances secreted by the body whose function is to stimulate the growth of the cells involved in wound healing and inflammation.
- The project will be focusing on two recombinant growth factors, for its potential application in wound healing.
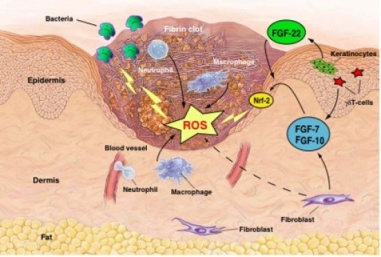
Phase Principal Investigator:
Dr. Anoop kumar T
Principal Clinical Investigator:
Dr. Padmakumar
Evaluation of biodegradable PLGC - fibrin hemostatic graft for skin regeneration
- Skin tissue engineering approaches using an electro spun biodegradable polymer scaffold with appropriate degradation profile and physico - chemical properties will be developed as a scaffold.
- Upon its implantation, undesirable contraction of the wound could be prevented.
Phase Principal Investigator:
Dr. Lissy Krishnan
Principal Clinical Investigator:
Dr. Prasanth Varkey
Atrial Septal Defect (ASD) Occluder
- An Atrial Septal Defect (ASD) is an opening or a hole in the wall that separates the two upper chambers of the heart.
- Conventional management technique is open heart surgery to close the hole.
- A special metal alloy (NiTiNOL) mesh is taken to the site using a catheter and then deployed to close the hole.
- Being a non - surgical procedure, the patient recovers fast and it requires a very short period of hospital stay.
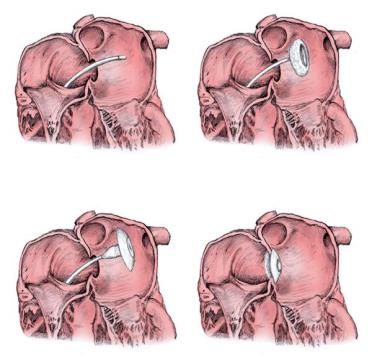
Phase Principal Investigator:
Mr. Sujesh S
Principal Clinical Investigator:
Dr. S Bijulal
Radiopaque Liquid Embolization Device
- Abnormal communication between small arteries and veins within the brain are described as arterio - venous malformation, causing seizures, intracranial bleeding, paralysis, visual loss or severe headache.
- Such defects are sealed with special sealants which set on contact with blood.
- A non-metallic sealant is planned in the project. This will help reduce MRI incompatibility.
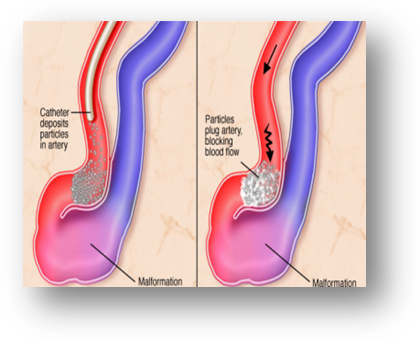
Phase Principal Investigator:
Dr. Roy Joseph
Principal Clinical Investigator:
Dr. Jayadevan E R
Bacillus Species producing molecule against Methicillin Resistant Staphylococcus Aureus (MRSA)
- MRSA is a bacterium that is tougher to treat because it is resistant to some commonly used antibiotics
- A microbial strain which produces an antibacterial agent which is active against MRSA has been isolated
- Project aims at detailed characterization of this antimicrobial molecule.
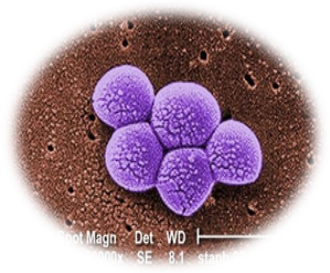
Phase Principal Investigator:
Dr. A Maya Nandkumar
Oral Insulin Delivery System
- The basic appeal of oral hypoglycaemic agents is that most people would prefer a pill or an oral liquid to an injection.
- However, insulin is a hormone, which is digested in the stomach and gut.
- The project attempts to develop a strategy for oral delivery of insulin by suitably encapsulating to protect it during its traverse through the stomach.
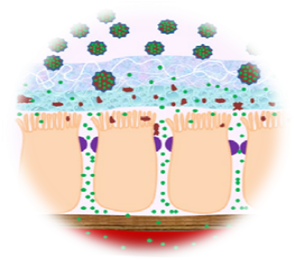
Phase Principal Investigator:
Dr. Rekha M R
Flow Diverter Stent
- Intracranial aneurysm is a condition in which weakness in the wall of a cerebral artery causes a localized dilation or ballooning of the blood vessel.
- Flow diversion is an endovascular technique whereby the device is placed in the parent blood vessel to divert blood flow away from the aneurysm itself.
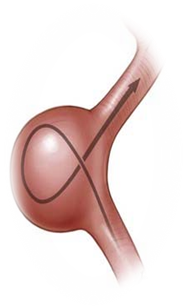
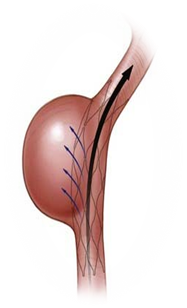
Phase Principal Investigator:
Mr. Sujesh S
Principal Clinical Investigator:
Dr. Santhosh K
Implantable Cardioverter Defibrillator
- An Implantable Cardioverter Defibrillator (ICD) is a battery-powered device implanted under the skin of the chest, which is able to perform cardioversion, defibrillation and pacing of the heart.
- Cardioversion is a procedure by which an abnormally fast heart rate (tachycardia) or other cardiac rhythm disorders are treated using electrical signals.
- Defibrillation is a treatment for life-threatening cardiac fibrillation (unsynchronized contraction of heart muscles) using electrical shocks.
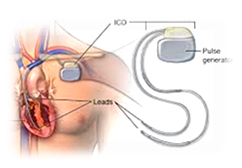
Phase Principal Investigator:
Mr. Muraleedharan C V
Principal Clinical Investigator:
Dr. Narayanan Namboodiri KK
Annuloplasty Ring for Mitral Valve Correction
- The Annuloplasty ring is a rigid or flexible ring implanted around the mitral valve for reconstructive treatment of valvular insufficiency.
- The implanted device enhances leaflet coaptation while preserving the native annular shape and motion.
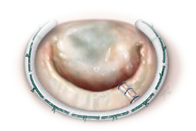
Phase Principal Investigator:
Mr. Ranjith G
Principal Clinical Investigator:
Dr. Vivek V Pillai
Programmable Hydrocephalus Shunt
- Hydrocephalus is the condition in which the cerebrospinal fluid (CSF) is accumulated within the brain. Due to this condition it may result in increased pressure inside the skull. It will result in various brain related problems especially in infants and older people. This condition is either due to birth defect or acquired later in the life. Hydrocephalus results in the conditions like Spina Bifida, Loss of consciousness, tiredness, irritability, drowsiness.
- Hydrocephalus is treated by a surgical operation in which a shunt which connects the brain region to other parts of human body. Generally the CSF is drained to the peritoneal cavity. In this case a shunt is called Ventriculo Peritoneal (VP) shunt. Another type of shunt is Ventriculo-atrial (VA) shunt in which the fluid moves to right atrium of the heart.
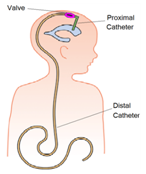
Phase Principal Investigator:
Mr. Anoop Gopinathan
Principal Clinical Investigator:
Dr. Easwer H V
Wound Healing Matrix composed of Human-fibrin, Amniotic membrane & Hyaluronic acid
- Combines three natural biological matrix components such as:
i. Fibrin- composite matrix which is hemostatic
ii. HA which hydrates the wound
iii. Amnion to use as an implantable biodegradable matrix to promote wound healing through guided tissue regeneration in vivo
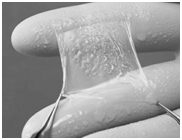
Phase Principal Investigator:
Dr. Lissy K Krishnan
Principal Clinical Investigator:
Dr. Prasanth Varkey
3 D Printing of Skin Tissue Constructs
- Aims to develop a skin construct
- A polymer ink based fabrication using 3D bio printing techniques used for encapsulating skin cells like Keratinocytes, fibroblasts and melanocytes for skin regeneration
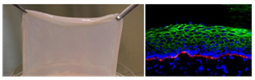
Phase Principal Investigator:
Dr. Anugya Bhatt
Principal Clinical Investigator:
-
Implantable Micro Infusion pump with Wireless Recharging System
- An implantable micro infusion pump which can deliver drug to the region of interest such as peritoneal cavity, spinal cord etc.
- Battery operated device will have a drug reservoir and a miniature drive mechanism for delivering the drug through a catheter.
- Refilling of drug can be achieved by injecting through a specific port.
- The device will be powered by a battery which can be recharged by transcutaneous energy transfer system (TETS) thereby avoiding percutaneous wires and associated infections
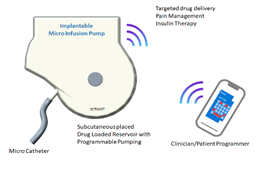
Phase Principal Investigator:
Mr. Sarath S Nair
Principal Clinical Investigator:
-
Parylene Coating For Medical Devices And Delivery Systems
- Parylene (poly paraxylylene) is a material used for coating medical devices and device delivery systems for imparting coatings with very low friction coefficient, good conformity and excellent dielectric properties.
- The projects aims to develop the parylene coating on different substrates which can be further extended in the fabrication of medical devices such as Left Ventricular Assist Device (LVAD), Deep Brain Stimulator (DBS) etc and for the delivery system for Aortic Stent graft, Atrial Septal Defect Occluder, Flow Diverter Stent etc.
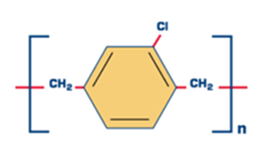
Phase Principal Investigator:
Dr. P. Ramesh
Principal Clinical Investigator:
-
Radiopaque Polymeric Microspheres For Embolization Therapy
- Embolization involves the selective occlusion of blood vessels by purposely introducing emboli and is used to treat a wide variety of conditions affecting different organs of the human body like aneurysm, tumor isolation etc.
- The objective of the project is to develop an indigenous technology for radiopaque polymeric microspheres intended for embolization therapy. The microspheres will contain covalently bound iodine as radiopacifying agent and polymer would be of non-degradable type
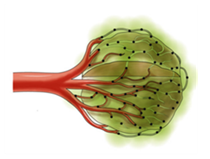
Phase Principal Investigator:
Dr. Roy Joseph
Principal Clinical Investigator:
Dr. Jayadevan E R
Titanium Nitride Coated Coronary Stent System
- Coronary artery disease (CAD) occurs when the coronary arteries are blocked with fatty deposits called plaque, which slowly builds up inside the artery causing them to narrow. This leads to shortness of breath, or chest pain or increased risk of heart attack. Coronary stents are usually made of metal alloy and are introduced on a balloon catheter in the crimped state into the lumen of the diseased artery. Later it is expanded by inflating the balloon at the point of implantation.
- This project aims to develop a ceramic coated stent system.
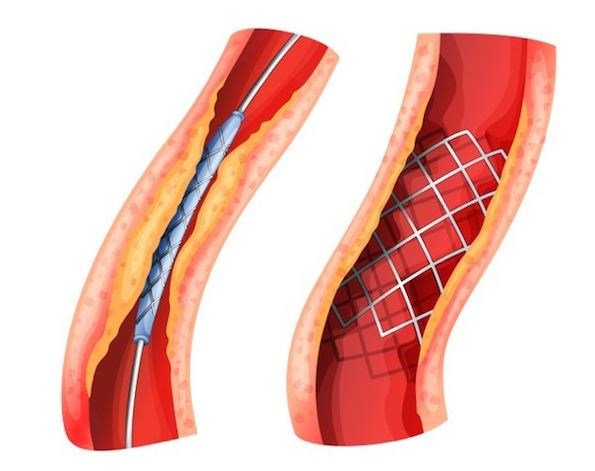
Phase Principal Investigator:
Mr. Subhash N N
Principal Clinical Investigator:
Dr. Harikrishnan S
Point of care detection of Human Papilloma virus using loop mediated amplification (LAMP) of DNA
- Develop a DNA based test
- Standardise LAMP based amplification of DNA as a point of care device for fast and cost effective detection

Phase Principal Investigator:
Dr. Anoop Kumar T
Principal Clinical Investigator:
Dr. Bindu Nambisan, SAT
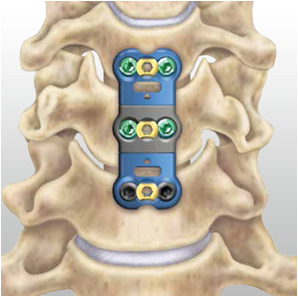
Spinal fixation system for thoracolumbar stabilization
- To design and develop novel Spinal fixation devices which include polyaxial pedicle screw, rod, locking cap, connectors and ergonomically designed instrumentation system.
- Optimize design parameters to make device construct sleek and to have variable sizes to suit paediatric patient’s population.
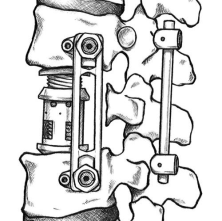
Phase Principal Investigator:
Mr. Arvind Kumar Prajapati
Principal Clinical Investigator:
Dr. Krishnakumar K
Development of high-strength Ti-6Al-4V (Ti64) castings for orthopaedic implants
- Develop a cost-effective process to produce high-strength Ti64 castings with tensile properties better than wrought, solution-treated and aged material
- Demonstrate material safety by conducting in vivo small animal model studies
- Demonstrate near-net, orthopedic implant prototype castings made using the process e.g. anterior-cervical plates
Phase Principal Investigator:
Dr. K G V Sivakumar
Principal Clinical Investigator:
Dr. Krishnakumar K
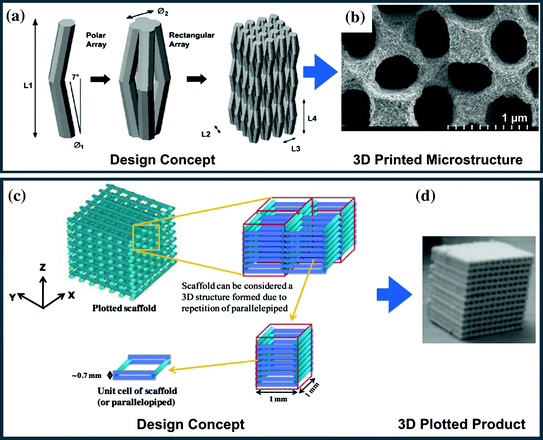
Bioceramic cages with axially aligned pores as a substitute for tricortical bone graft
- To design and develop a ceramic cage which is axially load bearing with aligned pores distributed around the central part.
- To investigate various mechanical and biological parameters of the ceramic cage for meeting essential clinical requirements.
- To evaluate mechanotransduction response of mesenchymal stem cells seeded on ceramic cages ex vivo using the bioreactor.
Phase Principal Investigator:
Dr. Manoj Komath
Principal Clinical Investigator:
Dr. Anoop Pillai, SUT
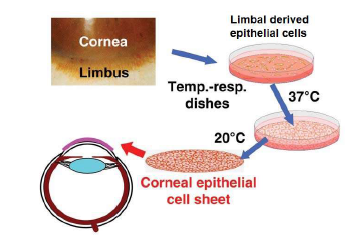
Corneal epithelial Cell Sheet Engineering: Validation and pre-clinical Evaluation
- Validation of thermo-responsive NGMA polymer synthesis.
- Validation of human corneal epithelial cell isolation and cell sheet generation.
- Pre-clinical Evaluation of human corneal epithelial cell sheet in rabbits
Phase Principal Investigator:
Dr. Naresh Kasoju
Principal Clinical Investigator:
Dr. Chitra Raghavan
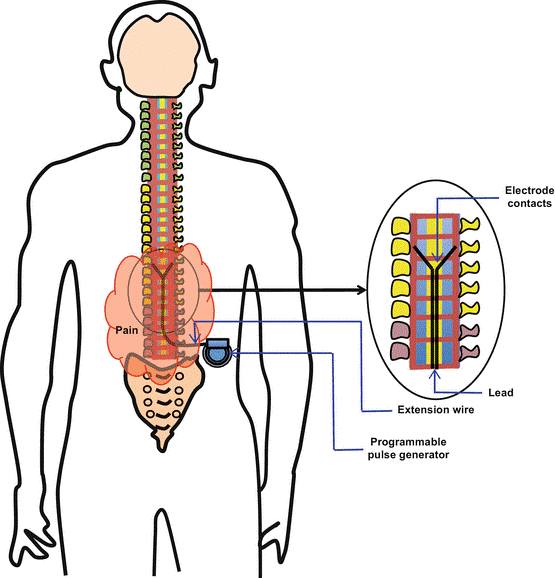
Development of a Spinal Cord Stimulator for pain management
- Chronic pain due to chronic neuropathic cases restricts mobility, interferes with normal functioning and results in lifelong pain and permanent disability.
- Spinal cord stimulation (SCS) therapy masks pain signals before they reach the brain.
- A small device similar to pacemaker called the implantable pulse generator (IPG) delivers electrical signals through specially designed electrodes into target areas in the spinal cord.
- It helps people better manage their chronic pain and reduce their use of opioid medications and improves the quality of life.
Phase Principal Investigator:
Mr. Jithin Krishnan
Development of Rapid Diagnostic Kits for Sepsis and Chlamydia Trachomatis
- Sepsis is a potentially life-threatening condition caused by the body's response to an infection. Sepsis occurs when the body's response to these chemicals is out of balance, triggering changes that can damage multiple organ systems.
- Chlamydia trachomatis is an intra cellular parasite which causes chlamydia, which can manifest in various ways, including: trachoma, urethritis, cervicitis and pelvic inflammatory disease.
- The project aims at developing lateral flow devices for detection of procalcitonin (PCT) for sepsis management and chlamydia trachomatis antigen for chlamydia management.

Phase Principal Investigator:
Dr. Manoj. G
FAST TRACK PROJECTS - COVID 19
Chitra Acrylosorb Fluid Solidification System
- The project aims at the development of superabsorbent material for body fluid solidification and disinfection.
- Acrylosorb can absorb liquids at least 20 times more than its dry weight and also contains a decontaminant for in situ disinfection.
- This technology reduces the risk for hospital staff, need for personnel for disinfecting and cleaning the bottles and canisters for reusing them and makes the disposal safer and easier.

Principal Investigator:
Dr. Manju S
Principal Clinical Investigator:
Dr. Ajay Prasad Hrishi,
Asso. Prof., Anaesthesiology
Licensed to M/s. Kerala State Drugs and Pharmaceuticals Ltd, Alappuzha
Chitra Emergency Breathing Assist System (Chitra EBAS)
- To develop a bridge ventilator for a few hours to few days in patients with moderate to severe breathing difficulty before conventional mechanical ventilation can be provided.
- The device enables positive pressure ventilation with controlled rate of expiration, I:E ratio, Tidal Volume etc.
- The automatic device will minimize the need of support personnel in the isolation room thereby enabling safer, effective and lung protective operation to Covid patients.

Principal Investigator:
Mr. Sarath S Nair
Principal Clinical Investigator:
Dr. Manikandan S,
Prof., Anaesthesiology
Technology transferred to M/s. Wipro Enterprises Pvt. Ltd, Bangalore
Digital Sanitization Systems
- To reduce the community spread of Covid � 19 viruses through contact surfaces, a low intensity burst of visible light or UV light in safe range controlled by a mobile app is explored.
- The flashlight (broad spectrum light) on the personal mobile smart phones is flickered at a rate for a certain duration using the mobile app. Alternatively, the UV light may be attached to the USB port of the mobile phone.
- The mobile may be focused to hands, or any objects to be sanitized and activate for some duration.

Principal Investigator:
Mr. Sarath S Nair
Chitra Isolation Pods
- The project aims at developing an isolation chamber to contain the patient and prevent the spread of infection during transportation and treatment in case of pandemic diseases.
- It provides safe isolation with internal negative pressure and the gases released by the patients is mitigated and neutralized.

Principal Investigator:
Mr. Sarath G
Technology transferred to M/s. HMT Ltd, Kochi
Relicensed to M/s. Kerala State Drugs and Pharmaceuticals
Emergency Response Isolation Systems
- The proposed concept utilizes locally available PVC pipes, connects and weather resistant fabric for setting up units in quick time with minimum kill and labour.
- deployable isolation wards, hospitals, ICUs

Principal Investigator:
Mr. Subhash N N
Technology transferred to M/s. Debrique Creative Labs Pvt. Ltd, Chennai
UV Based Disinfection Systems
- The proposed unit is for the sanitizing the facemasks prior to disposal thereby avoiding the potential spread of Covid - 19.
- It consists of a dustbin body, a controller and a UV disinfection lamp; which sterilizes and disinfects the used waste face masks prior to disposal.
Principal Investigator:
Mr. Subhash N N

UV based multipurpose disinfector
Licensed to :
- M/s. VST Mobility Solutions, Kochi.
- Commercially launched.

UV based face mask disinfection bin
Licensed to :
- M/s. VST Mobility Solutions, Kochi.
- Commercially launched.
Relicensed to
- M/s. Shivapriya Exim, Chennai
- M/s. PMG Equipment, Hyderabad
- M/s. Vivesty Green Recyclers, Kozhikode
- M/s. KSDPL, Alappuzha
Ventilator Sharing Kit
- The widespread shortage of ventilator equipment due to Covid 19 can be managed by sharing the single ventilator for multiple patients with the help of locally available devices.
Principal Investigator:
Mr. Vinodkumar V
Examination Booth
- To develop safe and efficient examination chambers to create engineering line of defense for healthcare professional who is examining Covid suspects.

Principal Investigator:
Mr. Ramesh Babu V
Technology transferred to:
- M/s. HLL Lifecare, Trivandrum
- M/s. Shivapriya Exim, Chennai
- M/s. HMG India, Mumbai
- M/s. TVS Supply Chain, Bangalore
- M/s. JADRO Steel, Kolkata
- M/s. KSDPL, Alappuzha
Chitra Swab Collection Booth
- To develop safe and efficient swab collection chambers to create engineering line of defense for healthcare professional collecting patient samples from Covid suspects.
- The proposed swab collection booth will also treat shed away contamination in upper compartment, which prevents possible surrounding contamination.

Chitra Single Chamber Swab Collection Booth

Chitra Dual Chamber Swab Collection Booth
Principal Investigator:
Mr. Ramesh Babu V
Technology transferred to
- M/s. HLL Lifecare, Trivandrum
- M/s. Shivapriya Exim, Chennai
- M/s. HMT, Kochi
- M/s. TVS Supply Chain, Bangalore
- JADRO Steel, Kolkata
- M/s. KSDPL, Alappuzha
- M/s. HMG India, Mumbai
Chitra Disinfection Gateway
- The Chitra Disinfection Gateway is meant for the decontamination of personnel entering a cleaner private space from a public space.
- This is equipped with an arrangement for generating hydrogen peroxide mist and ultraviolet rays. Hydrogen peroxide mist will decontaminate clothes, hands and the bags a person carries.
- The ultraviolet system will decontaminate the chamber once the person has moved out.


Principal Investigator:
Mr.Jithin Krishnan
Technology transferred to
- M/s. HLL Lifecare, Trivandrum
- M/s. Shivapriya Exim, Chennai
- M/s. HMT, Kochi
- M/s. HMG India, Mumbai
- M/s. TVS Supply Chain, Bangalore
- JADRO Steel, Kolkata
- INDIAHUB, New Delhi
- Technopark Hospital, Trivandrum
- Indian Railways, Trivandrum Division
Antigen detection kit for Covid 19
- To develop the diagnostic test kit which can detect the presence of Covid 19 antigen.
- Antibody against SPIKE protein will be immobilized on strips/stick and the kit will be developed for antigen detection based on lateral flow/ dipstick technique.
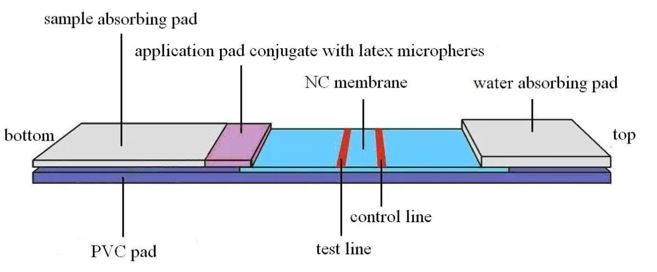
Principal Investigator:
Dr. Anugya Bhatt
Technology transferred to M/s. Agappe Diagnostics Ltd, Kochi;
Rapid Detection Kit for IgG/IgM Antibody
- To develop the diagnostic test kit which can detect the presence of antibody (IgM/IgG) specific to Covid 19 virus in serum, plasma or whole blood
- Enables to identify infection and isolate people without symptoms but Covid suspects.
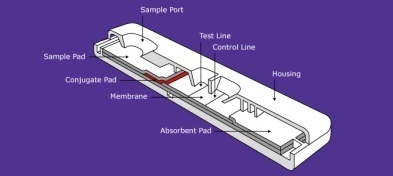
Principal Investigator:
Dr. Manoj. G
Technology transferred to Origin Diagnostics and Research, Kollam, Kerala
Nylon Flocked Swabs (Nasopharyngeal and Oropharyngeal) for Covid 19 testing
- The developed device contains nasopharyngeal and oropharyngeal swabs with a plastic shaft made of polypropylene having a breakpoint, with electrostatically flocked nylon for efficient collection of specimen.
- Two options either, throat or nasal are planned to be provided based on the site of specimen recovery. NP swabs can be used for testing asymptomatic persons.

Principal Investigator:
Dr. Lynda Thomas
Technology trasnfered to M/s. Mallelil Industries Pvt. Ltd, Kochi;
Oropharyngeal Sample Collection Kit
- Testing of Covid 19 involves collection of viral load in Viral Transport Medium (VTM) using a synthetic nasopharyngeal or oropharyngeal swabs.
- The project proposes development of Phosphate Buffered Saline (PBS) and Glycerol based VTM media along with the synthetic oropharyngeal swab for collecting the samples.
- This device can be used independently or as an accessory component for the sample collection kit for specimen collection from oropharyngeal region.
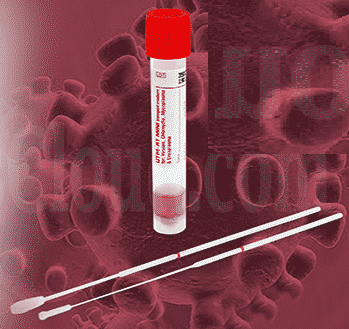
Principal Investigator:
Dr. Anugya Bhatt
Technology transferred to
- M/s. Origin Diagnostics and Research, Kerala
- M/s. Levram Life sciences Pvt. Ltd., Mumbai
Chitra LAMP-N RT-LAMP Kit and Chitra Magna RNA Isolation Kit
- Develop a diagnostic device and kit for rapid detection of Covid 19 virus using RT-LAMP technology.
- Develop a kit for RNA isolation from swab samples.
Principal Investigator:
Dr. Anoop Kumar T

Chitra Magna RNA Isolation Kit
- Technology transferred to M/s. Agappe Diagnostics Ltd, Kochi;
- Relicensed to M/s. Tata Sons Pvt. Ltd, Mumbai

Chitra LAMP-N Covid test kit
- Technology transferred to M/s. Agappe Diagnostics Ltd, Kochi;
- Relicensed to M/s. Tata Sons Pvt. Ltd, Mumbai
Development of a cost effective ventilator
- To develop a simple, compact, reliable and cost effective general purpose ventilator for adult and pediatric use on fast track mode.
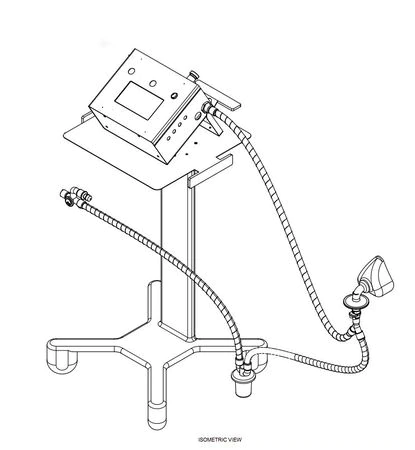
Principal Investigator:
Mr.Nagesh D S







